Instructions for Authors
- submission of Manuscript
- Plagiarism check
- During editing and reviewing steps
- Important dates to be considered
- Payment
submission of Manuscript
- Please read all the instructions on each page of the submission carefully.
- Create an account as an author. As a corresponding author, make sure that you write your name and your own email address that you would like to be reached
- Please click on the square beside "select if this is the Correspond Author" at the end of the authors page
- Select "Submit a new manuscript"
- Select Manuscript Type
- Follow the steps until attach files.
- In the step of "attach file", choose file from your computer then press "attach file". if you notice that attaching is taking a long time (more than one minute), you need to press on "attach file" again until you see your file on a table below.
- Attach files that have this symbol beside it (*). If the mandatory files are not attached, the website will not allow you to finish your submission.
- Please include your official email as a corresponding author.
- Add your supervisors/co-authors in the registration steps.
- Print the authorship form, sign it from your supervisors/co-authors and scan it , then upload it as an authorship form in JPEG format.
- Cover letter should include title of the manuscript, authors’ details.
- Follow the steps until you finish submission.
- All authors (whether they are supervisors or co-authors) should be included during submission as co-authors so that they can receive notifications during the whole process.
- In the step of " finish submission", review your details then press "submit manuscript"
- You'll receive email notifications for every step starting from submission till acceptance of the manuscript.
- When you receive corrections, click on ID number to see all corrections, download files sent back from Editor in chief and resubmit the corrected manuscript as “manuscript file”.
N.B: don't click on BACK button to go to a previous step. If you'd like to go to a previous step, press on the item on the side menu or "previous" button.
- The Title Page
- Authorship form
- Manuscript main file
- Manuscript should be in a regular one-column format, not a 2-column format.
- Font should be Times New Roman with font size of 12, space should be 1.15. All paragraphs should be justified with an indent.
- Main text should include the following sections: Introduction, Material and Methods, Results and Discussion. The article should finish with Conclusion, followed by Acknowledgements where necessary.
- Subtitles, (ABSTRACT, INTRODUCTION, MATERIAL AND METHODS, RESULTS, DISCUSSION, CONCLUSION, REFERENCES) should be in bold, uppercase, not underlined and not numbered.
- Please add the following as an attached file:
- Informed consent in case if the study is made on patients (not inside manuscript)
- Attach photo of the ethics committee approval among attachments
- Please add Ethics code number inside manuscript.
- Please add the following inside manuscript, after conclusion
- Recommendations
- Acknowledgments
- No conflict of interest
- If there is any fund received for this study
- All text should be in the form of paragraphs beginning with an indent, with no underlining in text.
- The journal is following Vancouver style.
- Figures should be kept to a reasonable number and they should be of high quality in JPG format with a size of 5x8.
- PNG format is not allowed and they should be transformed into JPG as follows:
- Write click on the figure and choose “save picture as”, then choose JPEG from options and replace the PNG format with JPEG.
- You should never manipulate your images to change or improve your results.
- Figures should be referred to in the text as (Fig. 1)
- Below the figure, the title of the figure should be written as illustrated in the example.
- Include scale bars
- Consider labeling important items
- Indicate the meaning of different colors and symbols used, either on the image or below it.
- Number, Title and Description of the figure is written clearly below the figure.
- Authors are responsible for obtaining permission to publish any figures or illustrations that are protected by copyright.
- Figures that do not meet these standards will not be accepted and may delay publication until we receive high-resolution images following the standards.
- All tables are preferably shaded as in the example.
- Title of the table should be clearly written above the table as illustrated in the example.
- Titles of columns and rows should be bold. However the rest of the content are not bold.
- All contents of the table is centralized.
- Statistical notes about the table are placed directly under the table as in example.
- Your recommendations should highlight specific solutions and measures based on the findings of your research.
- It should be concrete and specific for the problems you have stated earlier.
- The recommendations should connect to your conclusion.
- Acknowledgement is not a thank you note. Acknowledging people who contributed for the study establishes your integrity as a researcher.
- Only those who are not recognized as authors may be thanked and acknowledged. You should only quote those who contributed directly to your research other than co-authors
- Acknowledgement should be a simple statement of gratitude, not dedication or very emotional testimonial.
- • A brief acknowledgement is used to show contributions of different kinds; intellectual assistance, financial support, image credits, supply of animal subjects, cells, equipment setup, analytical/spectroscopy techniques,.. etc
- • You may mention following persons/institutions:
- Those who provided scientific guidance (other than supervisors and co-authors).
- Those who provided facilities & equipment.
- Technicians, lab assistants
- Funding agency, institution
- Students
- Maintain formal language, complete and professional sentences to Express respect and appreciation
- Titles such as Dr., Professor etc. are used. The institutions of the acknowledged people are mentioned.
- Use the complete names of individuals, funding agencies, libraries and other organizations.
- The author/s declare/s that he has no conflict of interest.
- The author/s declare/s that there is no conflict of interest
- None. All the authors declare no conflict of interest.
- The authors have no conflicts of interest relevant to this study.
- None Declared.
- If there was no funding received for the study, you can use the following wording:
- No external financial support was used.
- The authors received no specific funding for this work.
- There are no financial conflicts of interest to disclose
- The author(s) received no financial support for the research, and/or publication of this article from any funding agency
- If there was any kind of funding received for the study, you can use the following wording:
- This research is sponsored by [company name]
- - This work was supported by [institution name]
- At least 30% of the total number of references should be within the last 2 years.
- At least 70% of the total number of references should be within the last 5 years.
- References should be referred to in text, as a superscript between brackets before the full stop.
- Multiple successive references should be written with a dash; for example (14, 15, 16) should be written (14-16).
- In the reference section, references are written as follows: Citation of an article is written as: Author name, title of article, name of journal, year; volume: page number.
- Page number format example: write 556-9 (don not write: 556-559), write 1134-55 (do not write 1134-1155).
- Print journal article with six or fewer authors:
Example: Renton T, Van der Cruyssen F. Posttraumatic Trigeminal Neuropathic Pain in Association with Dental Implant Surgery. Dent Clin North Am. 2023; 67(1):85-98. doi: 10.1016/j.cden.2022.07.007.
- Print journal article with more than six authors:
If you have more than six authors, list the first six authors, followed by et al. - Payment should be processed at the publisher account, the Faculty of Dental Medicine for Girls, Al-Azhar University as follows:
Bank account No: 1433071120943801018
Bank: National Bank of Egypt
Beneficiary name: Al-Azhar Journal of Dentistry
Article processing charge: US $(200$) or Equivalent amount in EGYPTIAN pounds (there will be extra charge for print-outs inside Egypt)
Then Authors must upload a photocopy of the payment to their journal accounts. - https://pactr.samrc.ac.za/;
- http://www.clinicaltrials.gov/;
- http://www.ctri.nic.in/;
- http://www.anzctr.org.au/;
- http://isrctn.org/;
- http://www.trialregister.nl/trialreg/index.asp;
- http://www.umin.ac.jp/ctr.
- How far does this depart from the current standard (accepted, local) clinical practice?
- What additional burden is placed on the patients (or other parties)?
- What additional risks might patients (or others) face?
- What advantages might the patients (or others) experience?
- What possible advantages could there be for society (future patients)?
- Editors might ask authors for more specific information, like:
- how the authors defended the work's moral and ethical foundations.
- to give the journal the contact information for the research ethics committee that reviewed the work so that the committee can be asked for more details and justification
- For studies that have not been reviewed by research ethics committees or institutional review boards, to describe the ethical issues they took into consideration and how they justified their work
- It adheres to pertinent laws and regulations and follows institutional, national, and international standards for the humane treatment of animals.
- It has been approved by the ethics review committee at the institution or practice at which the studies were conducted (where such a committee exists);
- It demonstrates compliance with the institution’s ethics committee guidelines for research work involving non-human primates;
- It demonstrates a high standard (best practice) of veterinary care and involves informed client consent for studies involving client-owned animals
- Ensure that legal and ethical requirements related to the humane treatment of animals described in the study are met
- The Materials and Methods section presents the ethics committee approval process and the international, national, and/or institutional guidelines that were followed.
- The editors reserve the right to reject manuscripts based on ethical or animal welfare concerns. Research will be rejected on ethical grounds if the research involves unnecessary pain, suffering, distress, or permanent harm to animals, or if the severity of the experimental procedures is not justified by the value of the research submitted.
- Authors, not the journals nor the publisher, need to obtain the patient consent form before the publication and have the form properly archived. Patient consent should be written and archived with the journal, the authors, or both, as dictated by local regulations or laws.
- If the manuscript contains patient images that preclude anonymity, or a description that has obvious indication to the identity of the patient, a statement about obtaining informed patient consent should be indicated in the manuscript.
- If patients’ pictures are being used, the eye region should be masked to protect their anonymity.
The title page should include the title of the article, name of the corresponding author, email and full affiliation.
The authorship form should be obtained from the journal’s secretary or from the journal’s website, signed from all authors (whether they are supervisors or co-authors). An image (scanned or taken by a mobile) of an authorship form should be in JPEG format and should be uploaded as an authorship form. You can download the authorship form from here.
Title should be centralized and each word capitalized except for articles and prepositions (a, an, the, of, in, at, of,…etc).
Abstract: Provide an abstract containing 150 - 250 words. Briefly state the purpose, material and methods, results and conclusions. Subtitles within the abstract should be bold such as the illustrated example.
Keywords: authors are asked to provide up to five keywords, to be used as an aid to indexing.
Authors: information and Affiliations should be in the first page and will be added only after final reviewing. Affiliations should be referenced in text using superscript numbers without brackets as below. Names and affiliations of all authors should be correct as they wish to write them.
* Correct affiliation (place of work and position, city, country) should be written for each author as the example below:

Main Text:
Figures:
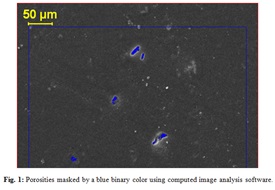
Figure 1: Porosities masked by a blue binary color using computed image analysis software
Tables:
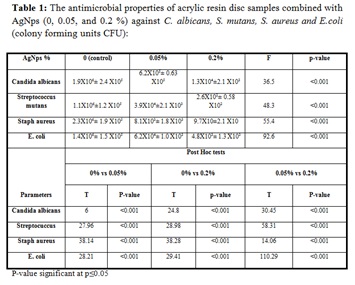
RECOMMENDATIONS
ACKNOWLEDGMENT
An example of the acknowledgment is shown below

References
ETHICAL APPROVAL
Please refer to the ethics section in the guidelines for details
Example: The study was approved by the Research Ethics Committee in your, Faculty of Dental Medicine for Girls, Al-Azhar University (approval no: ……………….)
CONFLICT OF INTEREST
Declaring conflicts of interest is very important for maintaining the honesty and integrity of unbiased professional assessment of the papers.
Examples:
FUNDING
Examples:
Examples:
References:
Examples:
de Resende GP, de Menezes EEG, Maniewicz S, Srinivasan M, Leles CR. Prosthodontic outcomes of mandibular overdenture treatment with one or two implants: 4-year results of a randomized clinical trial [published online ahead of print, 2023 Jan 24]. Clin Oral Implants Res. 2023;10.1111/clr.14036. doi:10.1111/clr.14036
Plagiarism check
Plagiarism has to be less than 20%. If your manuscript exceeds this percentage, it will not be accepted.
Payment
After accepting the manuscript:
Clinical trial registry
The journal would publish clinical trials that have been registered with a clinical trial registry that allows free online access to public. Registration in the following trial registers is acceptable:
This is applicable to clinical trials that commenced subject enrollment in or after June 2008. Clinical trials that started enrolling subjects before June 2008 would only be taken into consideration for publication in Al-Azhar Journal of Dentistry if they had been retroactively registered with a clinical trial registry that permits unrestricted online access to the public without charging any fees. a statement stating that the research was conducted with the ethical committee's approval and/or in one of the websites previously mentioned. The ethical code and the ethics committee's approval may be requested by reviewers, so it must be prepared.
Preparation of Manuscripts
Manuscripts must be prepared in accordance with the guidelines of Al-Azhar Journal of Dentistry. Before submitting a manuscript, contributors are requested to check for the latest instructions available. Al-Azhar Journal of Dentistry accepts manuscripts written in American English
Copies of any permission(s)
It is the authors'/contributors' duty to obtain permissions before duplicating any protected content. The manuscript must be submitted with a copy of the authorization received. The manuscript must be accompanied by copies of any and all related manuscripts that have been published, as well as any further manuscripts that are either in the works or have been submitted elsewhere.
Ethics:
By following the World Medical Association's Declaration of Helsinki, we anticipate all manuscripts published by Al-Azhar Journal of Dentistry to report on morally acceptable work. To do this, we evaluate the ethical implications of any submitted work that uses human subjects, regardless of the label that is used to describe it, including research, audit, and sometimes debate. Al-Azhar Journal of Dentistry Ethics Committee provided guidance and assistance in the development of our policy on these matters, and its essential components are discussed here.
Statement of Ethics Approval
Every research article that is submitted must include a statement that the study received ethics approval (or a statement explaining why it was not necessary), including the names of any institutional review boards or ethics committees involved, the approval number or ID, and a statement that participants provided informed consent prior to participating in the study.
Additionally, we want in-depth justifications of how researchers and authors have thought about and justified the ethical and moral foundation of their work. If this information cannot be included in the manuscript, please include it in the cover letter or upload it with the article as an additional file. Additionally, we will be happy to have copies of the explanation materials distributed to participants. Even if we decide not to include such specific material in the final version that is published, we might nevertheless provide it to editorial boards and peer reviewers. Peer reviewers are already requested to take the submitted work's ethics into account and offer feedback.
Appraisal of Ethical Issues:
In spite of the fact that this is undoubtedly one of the most crucial factors to take into account, editorial evaluation of ethical issues goes beyond simply determining whether study participants provided informed consent. As summed up by the following questions, editors should determine whether the overall design and conduct of each piece of work is morally justifiable.
What happens when the journal considers a study to be unethical?
In our opinion, editors have a responsibility to address questions of unethical audit or research, not to seek punishment for the authors but rather to stop unethical practice and to protect patients. If the Editor determines that the work in a submitted article is unethical, with or without the advice of its ethics committee, the Editor may seek additional guidance, recommend an investigation, or call for action. The fact that the article would have been rejected for other scientific or editorial reasons regardless would not stop the editor from taking such further action on serious ethics issues. In the first instance, the editor would typically get in touch with the head of the division where the work was done to express their concerns and suggest a local investigation. Second, the editor may send a letter to the guarantor or principal investigator of the paper's professional registration body.
Ethical approval of research involving animals
High ethical standards pertaining to animal welfare must be followed in all content reported in the Al-Azhar Journal of Dentistry that uses animal experiments.
Manuscripts will be considered for publication only if the work described that:
Before a manuscript is accepted, authors should:
Study design:
Participants Selection and Description: a clear description of the study participants (either patients or laboratory animals, including controls) of the observational or experimental studies, must be provided. The inclusion and exclusion criteria must also be provided together with the description of the source population.
Technical information: methodology should follow detailed description of all methods, and devices used (including the manufacturer's name and address in parentheses). Provide references to the mentioned methods, including statistical methods (see below); give references and brief descriptions for all technical information. Describe new or substantially modified methods, verify their usage, and assess their limitations. Clearly mention all chemicals and drugs used, comprising generic name(s), dose(s), and route(s) of administration.
Reports of randomized clinical trials should mention information on all major study elements, including the protocol, assignment of interventions (methods of randomization, concealment of allocation to treatment groups), and the method of masking (blinding), based on the CONSORT Statement (http://www.consort-statement.org).
Reporting Guidelines for Specific Study Designs
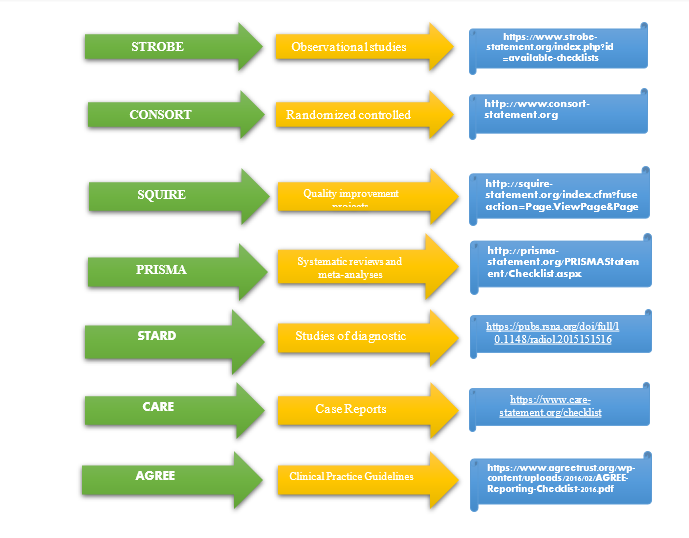
Statistics:
Study findings should be presented with suitable indicators of measurement error or uncertainty (such as intervals of confidence). Observational losses such as dropouts in clinical trials should be clearly addressed. When data are summarized in the Results section, specify the statistical methods used to analyze them. Avoid non-technical uses of technical terms in statistics, such as 'random' (which implies a randomizing device), 'normal', 'significant', 'correlations', and 'sample'. Mean differences in continuous variables, proportions in categorical variables, and relative risks including odds ratios and hazard ratios should be accompanied by their confidence intervals. For all P values include the exact value and not less than 0.05 or 0.001. Define statistical terms, abbreviations, and most symbols. Specify the computer software used. Use upper italics (P 0.048).
Protection of Patients' Rights to Privacy
All personal information should not be published in written descriptions, photographs, CT scans, sonograms, etc., and pedigrees unless the information is essential for scientific purposes and the patient (or parent or guardian, wherever applicable) gives informed consent for publication. All participants’ names should be removed from the figures except for informed consent. The journal abides by ICMJE (The International Committee of Medical Journal Editors) guidelines:
Sending a revised manuscript
The revised version of the manuscript should be submitted online in a manner similar to that used for the submission of the manuscript for the first time. Although, no need for submission of the “First Page” or “Covering Letter” file while the revision process is ongoing. When submitting a revised manuscript, contributors are requested to include, the ‘referees’ remarks along with point-to-point clarification at the beginning of the revised file itself. In addition, they are expected to mark the changes as underlined or colored text in the article.
Publication schedule
The journal is quarterly published with 4 issues per year and 1 volume per year. Issues are predominantly published in January, April, July, and October.








